UniPure’s patented iron co-precipitation process is the most efficient method of activating iron to indiscriminately remove all heavy metals from water or wastewater. Simply press start to see our process in action.
UniPure’s patented iron co-precipitation process is the most efficient method of activating iron to indiscriminately remove all heavy metals from water or wastewater. Simply press start to see our process in action. UniPure Process Technology was developed by the Science & Technology Division of Unocal to provide our industrial clients and remediation professionals with a simple treatment process that met stringent discharge criteria for heavy metals. The UniPure Process incorporates the following objectives:
- Provide a simple, flexible, highly effective metals removal mechanism
- Overcome the thermodynamic limitations of conventional hydroxide precipitation processes
- Can be used in applications that require part per billion effluent concentrations with multiple metal contaminants
- Can be used in industrial applications with highly variable metals loading rates
- Minimize overall Operation & Maintenance costs
- Produce stable, easily handled solids
- Can easily retrofit into existing systems to maximize their utility
The UniPure Process takes advantage of the many beneficial characteristics iron exhibits:
Natural Coagulation
Iron solids in water naturally coagulate without the addition of chemical coagulants. This helps to minimize flocculating polymer usage and greatly enhances the agglomeration of fines and colloidal material resulting in very low turbidity.
Natural Adsorbent
Heavy metals and many other substances often present in wastewater naturally adsorb to the surface of iron solids. This adsorption provides a degree of removal which can be beneficial, although the conventional adsorption of metals to iron is reversible.
Good Clarification and Dewatering
Iron solids are more dense than other metal hydroxide precipitates. This increased density allows the iron solids to settle more efficiently in a gravity clarifier and dewater more easily in a filter press. UniPure Process Technology makes use of, and improves upon all of these natural characteristics of iron, and incorporates several unique mechanisms to effect a superior metals removal technology.
Background
An understanding of the basic chemistry of conventional alkaline precipitation technology is essential to understanding the unique advantages of UniPure Process Technology.
Alkaline precipitation is still the most commonly used technology for removing of heavy metals from water. It is based on the occurrence of the following reaction:
![]()
In this equation, the M++ represents any divalent heavy metal. As shown, the metal ion combines with hydroxide ion to form the insoluble metal hydroxide solid. This reaction is pH dependent; as more base is added, the reaction is driven further to the right to precipitate more of the metals. Conversely, as the pH is decreased, the thermodynamic equilibrium moves to the left, causing more of the metals to resolubilize. This reaction is fully reversible and results in a solubility curve similar to that shown in Figure 1.
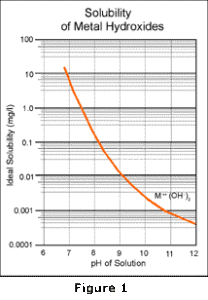
The limitations of alkaline precipitation technology result from secondary reactions which occur as more hydroxide is added. One common reaction is the combination of the metal hydroxide precipitate with additional hydroxide ion:
![]()
The metal hydroxide precipitate combines with additional hydroxide to form a soluble metal complex. Thus as the pH increases, the reaction proceeds to the right and the metal becomes more soluble. The solubility curve of this reaction approximately mirrors the curve of the first reaction as shown in Figure 2.
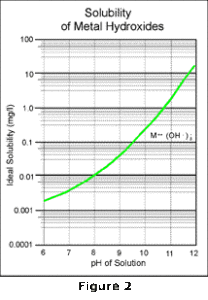
By overlaying these two curves as shown in Figure 3, and then combining the effects of all the
reactions which take place, the familiar V-shaped solubility curve for metal hydroxides is obtained as
shown in Figure 4.
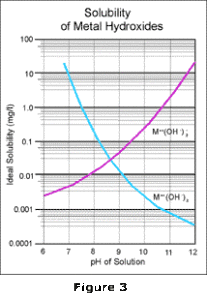
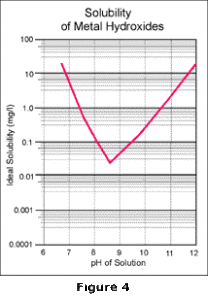
The lowest point of this curve identifies the absolute lowest concentration of a particular metal which can be achieved, under ideal conditions, by alkaline precipitation technology. To obtain this concentration, the pH must be maintained precisely at the indicated pH; any variation in pH will cause the metals to resolubilize. In a wastewater stream with multiple metals, this problem becomes even more serious because the low point occurs at a different pH for each of the metals. Any operating pH chosen is therefore a compromise; one metal may be at its lowest point, but all others will be elevated on their respective curves as shown by Figure 5.

UniPure Process Technology
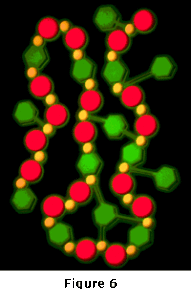
When ferrous iron (Fe+2) is added to water, the iron tends to form a soluble chain-like structure in solution due to the weak ionic attractions between molecules. Figure 6 shows a simplified example of such a chain. In a contaminated stream with heavy metals present, the heavy metals may be substituted into the chain in place of some of the iron atoms. This close association between the heavy metals and the iron, which occurs soon after the two are commingled, provides the mechanism by which the iron can be targeted to most efficiently remove the heavy metals. After the soluble association is formed, the water is introduced to the reaction zone of the UniPure Reactor.
In the reaction zone, base or acid is added to control the pH to 7.8 and air is sparged to rapidly oxidize the ferrous iron to ferric (Fe+3) iron. Through this rapid oxidation the solubility of the iron falls from the Fe+2 curve to the Fe+3 curve shown on Figure 5. As the iron drops out of solution, it incorporates the heavy metals in a dense iron matrix as it forms. The metals are “occluded” in the iron solids due to their close association with the iron prior to its precipitation. The occluded metals are effectively enveloped in iron; the solid presents itself to the surrounding solution as an Fe+3 particle and follows the Fe+3 solubility curve. The occluded metals are insulated from the solution by the iron, and so are not allowed to resolubilize as solubility curves in Figure 5 would indicate. This phenomenon makes it possible to remove heavy metals from water to concentrations well below their thermodynamic solubility limits.
Other metals which may not be removed by occlusion are removed by a second removal mechanism which operates simultaneously with the first. Two characteristics make this second removal possible:
- As mentioned earlier, ferric iron solids tend to adsorb heavy metals to their surface.
- Ferric iron acts as a catalyst for the oxidation of ferrous to ferric (i.e. the reaction is autocatalytic).
Ferric iron solids are present in the Reactor as a result of the oxidation of ferrous iron, and occasionally as a result of recycle from the clarifier. The solids, due to the very high shear environment of the UniPure Reactor, are highly fractured resulting in a very high surface area and many active adsorption sites. The heavy metals in solution adsorb to the surface of these solids in the Reactor. As ferrous iron enters the Reactor with the contaminated stream, the iron preferentially precipitates, due to the autocatalytic effect, on the solids which have already formed. This layering of iron precipitate on top of adsorbed heavy metals produces results similar to those achieved by the first mechanism; the heavy metals are effectively removed from solution and are isolated from the solution so that they cannot freely follow their solubility curves as the pH varies. This reaction mechanism also results in extremely dense solids since the precipitation is occurring directly on the surface of other solids. A slower precipitation of iron solids which are dispersed freely in solution produces a much less dense, and often colloidal mass of solids.
View UniPure Process Solids — Magnified Photos of Amorphous Iron Solids
View Solids Settling Demonstration
Discussion
The unique chemical mechanisms of UniPure Process Technology, combined with the many desirable characteristics of iron, result in a treatment process which is able to remove heavy metals to very low limits, and produces numerous other beneficial features:
- Hexavalent chromium is removed by UniPure Process Technology without the need for reduction pretreatment. As the ferrous iron is added to the contaminated water, the Cr+6 acts as the oxidant in place of the air. The hexavalent chromium is reduced to trivalent chromium as the ferrous iron is oxidized to the ferric state, and both are then effectively removed from solution as the UniPure Solids form.
- Any base may be used with UniPure Process Technology. The removal of heavy metals is dependent only on the chemistry of iron, and so the base may be chosen to minimize cost, minimize solids production, enhance recyclability of solids, minimize scaling, etc.
- Metals can be removed from water even in the presence of strong metal chelators. Chelating agents, which are present to varying degrees in almost all wastewater streams, are designed to act exactly contrary to the intent of a water treatment system; the chelators act to keep the metals in solution while the water treatment processes are intended to remove the metals from solution. UniPure Process Technology, because its removal mechanism is more physical than chemical, is able to remove chelated metals from solution by enveloping the metal and its associated chelator in iron and subsequently precipitating the iron solids. Processes which depend only on the thermodynamic equilibrium curves for removal have no such capability.
UniPure Process Technology produces solids which are extremely insoluble in water and mild acid solutions. The entrapped heavy metals cannot freely resolubilize so the results of leachability tests are significantly improved.The lower operating pH of UniPure Process Technology provides many benefits:
- Less base is required compared with conventional systems which often operate at pH in excess of 9.0.
- The lower operating pH translates to longer equipment life.
- The operating pH of UniPure Process Technology is within acceptable discharge limits, so no final pH adjustment station is required.
- A lower operating pH causes the solids produced to have a lower surface charge. This causes less water to be associated with the solids, hence better clarification with less settling area and better solids dewatering are achieved.
- The removal mechanisms of UniPure Process Technology are substantially non-selective. While heavy metals are removed most effectively, numerous other elements are entrapped and removed by the iron precipitate.
UniPure Process Technology can be implemented in two process configurations. The first, called the UniPure configuration, mirrors conventional treatment facilities. The UniPure configuration is best applied to treatment situations where the metals contamination is low or where the water has a high background iron concentration.
The second configuration, called Unipolish, takes effluent from a conventional treatment facility and passes it through a separate UniPure Treatment System. Unipolish is typically applied where existing conventional precipitation systems are not producing effluent which meets discharge standards. Polishing treatment with UniPure Process Technology is very effective at further removing metals concentrations.